Laboratories
 Brief description
Brief description
The experimental activities carried out at the MAMB laboratory in Messina are aimed at the ecological study of microorganisms by applying specific methods for the determination of the abundance and biomass of prokaryotes (Bacteria and Archaea) and phytoplankton, as well as for the morphometric and morphological description at the cellular level. The evaluation of these phenotypic characteristics provides a different approach to the analysis of the ecosystem structure and allows to evaluate the heterogeneity of natural populations. Variations in cell size, shape and morphology are considered sensitive indicators of trophic and climatic changes in ecosystems. The laboratory also carries out analyzes for the quantification of viable (Live / Dead) and respiring (CTC +) cells using specific microbial biomarkers. The laboratory activities are also in support of the EcoBiM and BiogeM Laboratories.
Matrices of interest
The matrices analyzed are mostly attributable to the hydrosphere (marine, river and lake waters, brines), soil, sediments, biofilm, cryosphere (permafrost, snow, sea and continental ice, intrapermafrost brines) and aquatic organisms.
Applied experimental approaches
- determination of biomass of prokaryotes by counting, and morphometric and morphological analysis of cells, using selective filters for DAPI (4 ', 6-diamidine-2-phenylindole);
- determination of cells with primary fluorescence using specific selective filters;
- quantification of cells endowed with respiratory activity (5-Cyano-2,3-ditolyl-tetrazolium chloride-CTC), using selective filters for rhodamine;
- quantification of viable cells with intact membranes (Live / Dead) using selective filters for fluorescein and rhodamine;
- identification of target bacterial cells by immunofluorescence techniques (fluoresceinated antibodies);
- estimation of the relative abundance of microbial phylogenetic groups using CARD-FISH (catalyzed reporter deposition-fluorescence in situ hybridization).
Instrumentation
- Zeiss AXIOPLAN 2 Imaging epifluorescence microscope equipped with AXIOCAMHR (Zeiss) digital video camera and AXIOVISION 3.1 software. Technical features: high pressure mercury vapor lamp (100 W); 100XPlan-Neofluar immersion objective; 10 X eyepieces, one of which has a squared reticle; interchangeable and appropriate optical filter sets for:
DAPI: G365 excitation, FT395 color divider and LP420 barrier filter;
Primary fluorescence: BP450-490 / FT510 / LP515;
Rhodamine: BP546 / 12; FT580; LP590;
Fluorescein: BP450-490; FT510; LP520.
For details: Sig. Giovanna Maimone – giovanna.maimone AT cnr.it
 Brief description
Brief description
The research activities carried out in the HydroChem laboratory in Messina are aimed at studying the marine (coastal and pelagic) and lake water bodies, providing technical-scientific support to the study of biogeochemical cycles and ecological processes also in relation to marine acidification, as well as studies of microbial biomass conducted in the MAMB Laboratory. In particular, analyses of oxygen, salinity, pH, nutrients (nitrogen and phosphorus) and particulate organic carbon are performed. As part of the environmental monitoring activity, several prototype nutrient analyzers were tested both on fixed platform and on boat, at pristine or anthropogenically polluted sites.
Matrices of interest
The analyzed matrices are mostly attributable to the hydrosphere (marine, river and lake waters, brines).
Instrumentation
The laboratory is equipped with basic instrumentation for environmental chemistry [spectrophotometer, spectrofluorimeter, luminometer, digital burette for oxygen titration, centrifuge, salinometer, automatic nutrient analyzers (ammonia, nitrites, nitrates and orthophosphates), extraction hoods, vacuum pumps, filtration septa, magnetic shaker, pHmeter].
For details: Dr. Filippo Azzaro – filippo.azzaro AT cnr.it
 Short description
Short description
The experimental activities carried out at the BioSoundEcology Lab in Messina are focused on the analysis of biological underwater acoustic sources and the study of the ecological dynamics of marine vertebrates and invertebrates in polar habitats. In addition, the impacts of anthropogenic acoustic sources on the ecology, physiology and behaviour of marine organisms are assessed. In particular, through the study of the sounds generated by animals, the biological and ecological aspects are investigated. The analysis of the noise from human activities provides useful elements for understanding the effects on animals through the study of their behavioural and physiological responses.
Matrices of interest
The activities are mostly ascribable to marine habitats.
Study techniques
At the BioSoundEcology Lab, acoustic data acquisition in field/controlled environment and assessment of animal responses to environmental noise disturbance are carried out, in particular:
- Acoustic data analysis;
- Estimation of underwater noise and environmental acoustic components;
- Evaluation of ecological dynamics through the analysis of the vocalizations of marine animals;
-Analysis of the biochemical, physiological and behavioural responses of animals exposed to acoustic disturbance;
- Study of the acoustic ecology of marine mammals.
Instrumentation
The Lab is equipped with instrumentation for passive acoustic monitoring in the field (hydrophones and autonomous acoustic data acquisition systems) and for studies of the effects of noise on marine organisms in controlled environment (cameras, amplifier, acoustic transducer, software for acoustic and behavioral analysis).
For details: Dr. Francesco Filiciotto – francesco.filiciotto AT cnr.it
 Short description
Short description
The research activities carried out in the BiogeM laboratory at the Messina headquarters are aimed at studying the marine and terrestrial biological processes that modulate and influence the chemical characteristics of the polar environment and the related cycles of matter and energy, also in relation to climate change.
Particular attention is paid to the evaluation of the role of microorganisms in the global carbon cycle and in the biogeochemical cycles of nutrients (nitrogen, phosphorus) of marine and lacustrine environments, through the study of both productive and degradative processes.
Among these, the activities involved in the decomposition of organic polymers, through microbial enzymatic activities, and the mineralization processes through microbial respiration.
The biogeochemical processes mediated by the microbial component are also being studied in the framework of the cryosphere, in order to understand the ecological significance of microbes in the permafrost, and their ability to maintain an active metabolism in extreme living conditions.
Together with the microbial processes linked to the biological pump and organic matter decomposition, a series of indirect biogeochemical parameters related to phytoplankton and bacterial biomass (Chlorophyll, ATP, lipopolysaccharides-LPS) are also determined.
Matrices of interest
The analyzed matrices are mostly ascribable to the hydrosphere (marine, river and lake waters, brines), soil, sediments, biofilm, cryosphere (permafrost, snow, sea and continental ice, intrapermafrost brines) and aquatic organisms.
Study techniques
At the BiogeM laboratory, measurements are carried out to determine the following parameters:
- Primary phytoplankton production;
- Microbial respiratory activity (consumed O2; produced CO2) (by ETS assay);
- Microbial enzymatic activities (leucin aminopeptidase, beta-glucosidase and alkaline phosphatase) (through fluorogenic substrates);
- Heterotrophic bacterial production;
- Content of total and size-fractionated (pico-, nano- and micro-phytoplanktonic) chlorophyll-a, pheopigments;
- microbial ATP in pico-, nano and microplankton fractions;
- Quantitative analysis of lipopolysaccharides (LPS) by the LAL chromogenic test.
Instrumentation
Spectrofluorimeter, Luminometer, Fluorometer, Spectrophotometer equipped with 96-well plate fluorescence reader, incubator, autoclave, balance, homogenizer, filtration systems, refrigerated centrifuge.
For information: Dr. Gabriella Caruso – gabriella.caruso AT cnr.it
 Brief description
Brief description
Experimental activities carried out at the EcoBiM LAB in Messina are addressed to the ecology and biotechnology of microorganisms, particularly prokaryotes, inhabiting both marine and continental polar habitats. The diversity of microorganisms, their response to environmental stress conditions (deriving from natural or anthropogenic forcing, such as climate change and chemical contamination), the astrobiological implications of life in extreme environments, and their evolution and adaptation in polar environments are among the ecological aspects investigated. EcoBiM researchers are also interested in the evaluation of the metabolic capabilities and biotechnological potentialities of cold-adapted microorganisms, by searching for biomolecules exploitable in industrial applications and bacteria able to degrade organic pollutants at low temperatures.
Matrices of interest
The analyzed matrices are mostly attributable to the hydrosphere (marine, river and lake waters, brines) and cryosphere (permafrost, snow, sea-ice and continental ice, intrapermafrost brines), but soils and sediments are also considered. The study of the interactions between microorganisms and biotic (for example, pelagic and benthic organisms) and abiotic (such as microbial communities colonizing polymeric materials, indicated with the term plastisphere) is of particular interest.
Applied experimental approaches
For the microbiological characterization of extreme environments, similarly to the analytical procedures commonly applied for the study of temperate areas, we use both culture-dependent and -independent (i.e. biomolecular and biochemical) approaches, including:
- isolation and maintenance in pure culture of bacterial strains;
- phenotypic (physiological, biochemical and morphological characteristics) and genotypic (analysis of the 16S rRNA sequences and search for functional genes) characterization of cultivable bacteria;
- screening of bacteria for the production for useful biomolecules (including antibiotics, exopolysaccharides, biosurfactants);
- evaluation of the metabolic capacities of the microbial communities through miniaturized assays;
- characterization of the microbial communities by the hybridization in situ with oligonucleotide probes (CARD-FISH);
- extraction of environmental DNA and RNA for metagenomics and metatrascriptomics studies;
- preparation of microcosms enriched with organic and inorganic contaminants, and degradation tests.
Instrumentation
The EcoBiM LAB is equipped with basic instrumentation for environmental and applied microbiology (laminar flow cabinet, autoclave, incubators and thermostated baths, centrifuges, filtration systems, sonicators, spectrophotometers, fluorometers) and molecular biology equipment (thermocycler and equipment for electrophoresis).
For details: Dr. Angelina Lo Giudice - angelina.logiudice AT cnr.it

High Performance Liquid Chromatograph Agilent 1100 Series HPLC Systems
Brief description
The HPLC-MS/MS system allows to quantitatively determine polar organic compounds in several environmental and vegetable matrices and biota samples. It is commonly used to investigate specific markers of sources or environmental processes in samples collected in polar regions. It is used to determine levoglucosan, key tracer of biomass burning, in ice core samples to provide historical profiles of fire regimes in paleoclimatic studies. Several water soluble organic compounds (free and combined amino acids, phenolic compounds) are determined using HPLC-MS/MS in aerosol samples to define chemical composition of atmosphere in urban and polar samples. Polar pesticide or toxins are commonly determined with HPLC-MS/MS in fresh water, sea water, biota or vegetable samples.
Instrument
High Performance Liquid Chromatograph Agilent 1100 Series HPLC Systems (Waldbronn, Germany) with a binary pump, vacuum degasser, autosampler and thermostated column compartment coupled with an API 4000 Triple Quadrupole Mass Spectrometer (Applied Biosystem/MSD SCIEX, Concord, Ontario, Canada) using a TurboV source.
Contact person: Dr. Roberta Zangrando - roberta.zangrando AT cnr.it - CNR-ISP Venice Headquarters
Matrix and type of measurement
Analysis of discrete samples of several matrices: ice, snow, atmospheric aerosol, lacustrine water, fresh water, sea water, sediment, vegetable and biota samples. Analysis of polar organic compounds such as for example anhydrosugars, amino acids, phenolic compounds, organic acids.
Agilent 1100 series HPLC system coupled with API 4000, High Performance Liquid Chromatograph coupled with tandem mass spectrometer (HPLC-MS/MS). (IMAGE)

Liquid chromatograph UHPLC mod. Dionex Ultimate 3000 Dual Pump RS
Brief description
This instrument is the key system to perform semicontinuous analysis of organic compounds in ice core samples.
Instrument
Liquid chromatograph UHPLC mod. Dionex Ultimate 3000 Dual Pump RS (Thermo ScientificTM) with vacuum degasser, column thermostat.
Contact person: Dr. Elena Barbaro - elena.barbaro AT cnr.it - CNR-ISP Venice Headquarters
Matrix and type of measurement
Semicontinuos analysis of ice cores. This system is coupled with continuous flow analysis (CFA).
Dionex Ultimate 3000 Dual Pump RS Thermo Scientific, Liquid chromatograph UHPLC Dual Pump (IMAGE)

Mercur Plus - Analytik Jena AG, cold vapor atomic fluorescence spectroscopy (CV-AFS).
Brief description
Cold vapor atomic fluorescence spectroscopy (CV-AFS) is an analytical technique used for the quantification of mercury at trace/ultra-trace levels. This technique is mainly used on “clean” aqueous matrices (eg ice, snow, and water) from remote and uncontaminated areas.
The sensitivity of the instrument is fully harnessed by using official methods such as USEPA1631 version E or UNI-EN 15853: 2010. The Hg present in the matrix is oxidized to Hg2+ with BrCl solution and then reduced to elemental mercury(Hg0) con SnCl2. The Hg0 is stripped from the aqueous matrix using an inert carrier gas (argon) and successively transported to gold traps for the pre-concentration by amalgam formation. Following thermal desorption at T between 450-500 °C, the Hg0 is desorbed from the gold traps and is transported into a quartz cell.
Light from a mercury vapor lamp passes through the quartz cell that contains the sample mercury in a flow of argon carrier gas and excites all the mercury atoms which then emit a characteristic fluorescence radiation at 253.7 nm. The amount of light emitted by the mercury atoms in the sample is proportional to the amount of mercury passing through the quartz cell. The CV-AFS of the CNR-ISP is located inside a dedicated clean room.
Instrument
Mercur Plus - Analytik Jena AG, cold vapor atomic fluorescence spectroscopy (CV-AFS).
Contact person: Dr. Massimiliano Vardè - massimiliano.varde AT cnr.it - CNR-ISP Venice Headquarters
Matrix and type of measurement
Aqueous matrices: atmospheric deposition, snow, ice, drinking water, mineral water, natural water, seawater. Mercury, as total mercury, or as dissolved, filtered and unfiltered after sample pre-treatment.
Mercur Plus - Analytik Jena AG, cold vapor atomic fluorescence spectroscopy (CV-AFS). (IMAGE)

Gas chromatograph coupled with mass spectrometer (GC-MS)
Brief description
The GC-MS system allows the quantification of volatile apolar compounds in environmental matrices. In the environmental field GC-MS finds main application in the determination of persistent organic pollutants (POPs) such as PCBs, PBDEs, PAHs, pesticides, in environmental matrices both in urban and remote areas such as polar areas. As well as it used in the determination of personal care products such as fragrances that have been observed not only in urban areas but also in Antarctica.
In the ISP-CNR there are 3 GC-MS systems. Among these, the system equipped with a cryogenic trap allows the preconcentration of volatile compounds (allowing the quantification of volatile compounds difficult to analyze in GC even at very low levels). There is also a GC-MS system equipped with a pyrolyser that allows the analysis of non-volatile materials such as plastic materials.
Instrument
• GC-MS 7890A-5975C (Agilent) /
• GC-MS GC7890A+MS5975C (Agilent) with cryogenic trap (MARKES Int)
• GC-MS GC6890+MS5973 (Agilent) with Pyrolysis system Pyroprobe 5000 Series
Contact person: Dr. Elena Argiriadis - elena.argiriadis AT cnr.it - CNR-ISP Venice Headquarters
Matrix and type of measurement
Analysis of discrete samples of several matrices: ice, snow, atmospheric aerosol, lacustrine water, fresh water, sea water, sediment, vegetable and biota samples. Compounds analyzed: Apolar volatile compounds such as PCBs, PBDEs, PAHs, pesticids, fragrances, sterols.
GC-MS 7890A-5975C Agilent (IMAGE)

Brief description
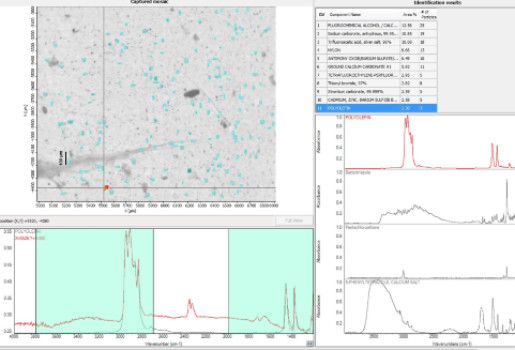
Microplastics are considered emerging pollutants and they are present in different environmental compartments (e.g. seawater, soil, atmosphere, etc.). In 2019 the European Chemical Agency has clearly defined microplastics and their sizes: “a material composed of solid polymer-containing particles, to which additives or other substances may have been added, with particle dimensions ranging from 1 nm to 5 mm and with fiber lengths ranging from 3 nm to 15 mm and length to diameter ratio of >3. ECHA has also firmly stated the need of polymer identification when analyzing microplastics. Microplastics can be primary and secondary, according to their sources; sources of primary microplastics are discharges from household washing machines, road dust, tire wear and cosmetics. Particles and fibers of plastics can be vector of other pollutants and pathogens. An accurate quantification and characterization of microplastics allow evaluating the environmental risk assessment and designing future actions of environmental management and recovery.
Instrument
Micro-FTIR Nicolet™ iN™10 Infrared Microscope Thermo Scientific, it couples optic microscopy with IR spectroscopy. Two different detectors are present: DTG (Deuturate Triglycine sulfate) detector enables room temperature analysis, and MCT (Mercury Cadmium Telluride ) detector works with liquid nitrogen and allows the analysis of samples down to 10 µm. Samples can be analyzed on transmittance mode, reflectance mode and ATR mode.
Matrix and type of measurement
Non-destructive analysis of microplastics in different environmental samples (seawater, sediments, soil, permafrost, aerosol, snow, raw and treated water, discharges from household washing machines, etc.) and in different biota (crustaceans, mollusks, fish, etc.). Micro-FTIR allows quantification and simultaneous polymer identification of plastic particles and fibers and of additives, plasticizers. and other synthetic and natural fibers. It can be employed for analysis of microplastics, but also for analysis of microfossils, artistic handiworks, etc.
Contact person: Dr. Fabiana Corami - fabiana.corami AT cnr.it - CNR-ISP Venice headquarters
Mass Spectroscopy Laboratory (SpeM)
Brief description
In the SpeM laboratory, at the Venice headquarters, the researchers carry out the chemical (trace elements) and isotopic (δD, δ13C, δ15N, δ18O) characterization of natural matrices and the determination of the total carbon and nitrogen content (in soils and sediments).
Trace elements are all those elements in the periodic table present in natural matrices with concentrations of less than 1 ppm (parts per million). The quantification of these elements allows, in addition to the chemical characterization of the samples examined, an evaluation of any contamination problems, including those arising from human activities. This allows us to study the exchanges that may take place between different compartments of the different ecosystems, and the origin of the elements, as well as allowing us to reconstruct temporal variations related to current and past climate changes.
Per Isotopes are atoms belonging to the same chemical element, therefore with the same atomic number but which differ by mass number (that is, they differ by the number of neutrons contained within the nucleus). Isotopes are therefore chemically equal but different from a physical point of view. They differ in nuclear stability and instability meaning that some can be radioactive. Among the most studied stable isotopes are oxygen, carbon, hydrogen and nitrogen. The study of stable isotope relative abundances between the various isotopes of an element, gives a measure of the ratio of one of the heavier isotopes to the lighter isotope (the most abundant in nature) of a given element. This ratio in nature is not constant but may vary as a result of chemical, physical and biological processes that can lead to an impoverishment or an enrichment of one isotope compared to the other in the various phases of a natural system: we speak of this as isotopic fractionation. Stable isotopes have fundamental applications for environmental and paleoenvironmental studies. For example, the measurement of δ18O in snow/ice cores allows a reconstruction of the temperature trends in the past.
Instrument



Element XR DeltaV Advantage Elemental Analyzer Flash2000HT
ICP-SFMS Element XR Thermo Scientific equipped with various sample introduction systems (Scott-type spray-chamber in PFA; cyclonic spray-chamber in glass or PFA, cooled by a Peltier system; APEX-ESI system equipped with heated cyclonic spray-chamber (both in glass and in PFA) and a cooling system for the reduction of interferences from oxides and doubly charged ions, resistant to hydrofluoric acid; ARIDUS-CETAC system equipped with heated spray-chamber in PFA and a heated membrane, for the reduction of oxides and doubly charged ions). The instrument is also equipped with an auto-sampler protected by a laminar flow hood that keeps the samples clean during the analysis sessions.
Contact person: Dr. Giulio Cozzi - giulio.cozzi AT cnr.it - CNR-ISP Venice headquarters
IRMS DeltaV Advantage Thermo Scientificequipped with: Gas-Bench (with autosampler), Elemental Analyzer Flash HT with double furnace, two autosamplers for solid samples and one autosampler for liquid samples and ConFloIV, and Gas chromatograph (GC Trace with auto-sampler).
Contact person: Dr. Clara Turetta - clara.turetta AT cnr.it - CNR-ISP Venice headquarters
EA Elemental Analyzer Flash2000HT Thermo Scientific with double furnace, two autosamplers for solid samples and one autosampler for liquid samples.
Contact person: Dr. Clara Turetta - clara.turetta AT cnr.it - CNR-ISP Venice headquarters
Matrix and type of measurement
ICP-SFMS: snow/ice, sea water, surface and groundwater, interstitial water. Determination of trace elements (at ppb to ppq level).
IRMS - Gas-Bench: water (snow/ice, sea water, surface and groundwater, interstitial water), carbonates (foraminifera, carbonates s.s.). δD, δ13C, δ18O measurements,
IRMS - EA: soils, sediments, biota. δ13C, δ15N measurements.
EA: soils, sediments, biota. Determination of total nitrogen (TN), total carbon (TC) and organic carbon (OC).
The Institute can provide paid services, in compliance with the current Regulations for the Generation, Management and Enhancement of Intellectual Property on the results of CNR research, for services on behalf of third parties (Contractual research).
The laboratories of the Institute of Polar Sciences carry out chemical, chemical-physical, microbiological analyzes on various environmental matrices, issuing the relevant certificate or technical / scientific reports.
The analyzes are performed ONLY UPON REQUEST sent by email to the address of the Laboratory / Instrumentation Contact and in cc to the Director of the Institute (direttore.isp AT cnr.it) for the Venice headquarters and the secondary site of Padua or to Head of branches (responsabile_bo AT cnr.it; responsabile_me AT cnr.it; responsabile_rm AT cnr.it) for the secondary sites of Bologna, Messina and Rome, containing the name, address and signature of the Applicant. The analyzes to be carried out, the number of samples and everything else to be requested must be listed.
By the mere fact of submitting the request, the rules and consequent charges are understood to be known and accepted by the Applicant. After receiving the request, the Contact Person, having obtained the clearance from the Director / Head of the branches, will send a cost quotation and indicate an expected date of delivery of the results.
The samples to be analyzed must be sent, free of all charges, to the branches of the contact person according to the methods indicated in the quotation. The rates that will be communicated are net of VAT and include the execution of the analyzes and the sending of a form containing the related results. No interpretation and / or evaluation of the analysis results will be made by the laboratory involved.
 In the Organic Geochemistry Laboratory at the Institute of Polar Sciences in Bologna, researchers and students deal with modern processes and paleo reconstructions by coupling the information provided by fossil biomarkers and stable carbon and nitrogen isotopes. Biomarkers and stable isotopes are geochemical proxies used to investigate the feedback mechanisms between the Earth climate and the biogeochemical cycles. The laboratory is equipped with various instruments to extract, purify and analyze a suite of different biomarkers, including terrestrial compounds (e.g. lignin phenols, aliphatic chain lipids, cutin-derived products) to understand land-ocean carbon exchange (e.g. permafrost thawing, river floods, etc.), alkenones for paleo-temperature reconstructions and highly branched isoprenoids for sea ice reconstructions. In addition, the laboratory is equipped with a Preparative Fraction Collector (Agilent-Gerstel) for the collection of individual compounds. This technique is especially useful for the radiocarbon analysis of biomarkers and can be very useful to derive an age model for the sedimentary archives and to investigate the processes related to permafrost thawing.
In the Organic Geochemistry Laboratory at the Institute of Polar Sciences in Bologna, researchers and students deal with modern processes and paleo reconstructions by coupling the information provided by fossil biomarkers and stable carbon and nitrogen isotopes. Biomarkers and stable isotopes are geochemical proxies used to investigate the feedback mechanisms between the Earth climate and the biogeochemical cycles. The laboratory is equipped with various instruments to extract, purify and analyze a suite of different biomarkers, including terrestrial compounds (e.g. lignin phenols, aliphatic chain lipids, cutin-derived products) to understand land-ocean carbon exchange (e.g. permafrost thawing, river floods, etc.), alkenones for paleo-temperature reconstructions and highly branched isoprenoids for sea ice reconstructions. In addition, the laboratory is equipped with a Preparative Fraction Collector (Agilent-Gerstel) for the collection of individual compounds. This technique is especially useful for the radiocarbon analysis of biomarkers and can be very useful to derive an age model for the sedimentary archives and to investigate the processes related to permafrost thawing.
Facilities
The Organic Geochemistry Laboratory is equipped with several gas chromatographers and mass spectrometers for the analysis of carbon and nitrogen stable isotopes, measurement of carbon, hydrogen, nitrogen and sulphur content in organic matter and the extraction/quantification of organic biomarkers. The analytical facilities include:
- Thermo Fisher Scientific FLASH 2000 Element Analyzer coupled with a mass spectrometer DeltaQ (EA-IRMS)
- GC Agilent GC 7820-MSD EI 5977B
- GC Agilent 8860-FID G2790A
- GC Agilent 8890 equipped by a Gerstel Preparative Fraction Collector (PFC)
For more information: Dr Tommaso Tesi - tommaso.tesi AT cnr.it
More...
Brief description
The experimental activities carried out at the BioChem Lab in Rome are focused on the study of the effects of legacy and emerging organic micropollutants on organisms belonging to different levels of the trophic web (from microbial communities to higher organisms) in the context of ongoing climate change. The analytical determinations on the biota are aimed at evaluating bioaccumulation and biomagnification processes of target pollutants. Other studies performed at the BioChem Lab focus on:
- The degradation rates (evaluation of the half-life time - DT50 - and determination of metabolites or transformation products of target compounds) of the organic contaminants by setting up laboratory-scale experiments.
- Evaluate the effects of organic micropollutants on specific target organisms (e.g. avoidance behaviour test in Eisenia foetida) and on the structure (PhosphoLipid Fatty Acid analysis-PLFA) and function (Community Level Physiological Profile - CLPP) of autochthonous microbial communities, through the preparation of laboratory-scale experiments and environmental monitoring.
- Investigate the occurrence and diffusion of antibiotic resistance genes (ARGs) and the intI1 gene which is an anthropogenic impact proxy, among natural microbial communities related to the presence of antibiotics in environmental matrices.
Environmental compartments
The bioaccumulation/biomagnification studies of organic micropollutants involve biota such as fish, molluscs, terrestrial and aquatic earthworms and terrestrial and aquatic plant species. The studies on persistence and effects of pollutants on natural microbial communities are related to different environmental compartments such as surface waters and sediments (seas, rivers, lakes), snow/ice and soils.
Analytical techniques
Studies on bioaccumulation/biomagnification/persistence of organic contaminants are performed by a combination of:
- Pre-treatment methods (e.g. freeze-drying, filtration, etc.).
- Extraction/Clean-up methods (solid-phase extraction-SPE, pressurized liquid extraction-PLE, liquid-liquid extraction-LL).
- Sensitive and selective analytical methods based on the coupling of chromatographic techniques (HPLC or GC) and fluorescence, FID-ECD, and mass spectrometric (MS) detection.
- The avoidance behaviour test is based on the exposure of organisms, specifically earthworms belonging to the Eisenia foetida species, to different sub-lethal concentrations of organic contaminants, to evaluate their escape/avoidance behaviour in the contaminated area.
- The evaluation of the composition of natural microbial communities is carried out by PLFA (PhosphoLipid Fatty Acid) profiling. This biochemical technique includes the extraction of fatty acids from microbial membrane phospholipids in the environmental matrix and a methylation reaction followed by separation and analysis with gas chromatography.
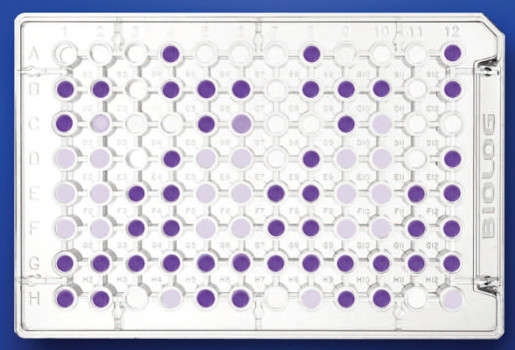
- The analysis of microbial diversity at a functional level is performed by the determination of the metabolic/physiological profile (CLPP). This physiological assay involves the incubation of the environmental sample in specific plates and spectrophotometric measurements at fixed times.
- The analysis of ARGs and intI1 gene is performed by DNA extraction from the environmental samples by using specific kits and subsequent analysis by qPCR.
Equipment
 The BioChem Lab is equipped with the following analytical tools:
The BioChem Lab is equipped with the following analytical tools:
Benchtop lyophilizer (freeze-dryer LABCONCO) 2.5 L capacity, equipped with a touchscreen display, for the pre-treatment of solid matrices subsequently extracted with PLE.
Solid Phase Extraction (SPE):12 inlets of the solid phase extractor are connected to cartridges packed with specific adsorbents for the extraction of target compounds from liquid matrices through a vacuum system.
Syncore® Analyst (Buchi) for the simultaneous pre-concentration of liquid phase samples.
Rotavapor R 100 (Buchi), equipped with an electronic interface to control the vacuum system and the recirculating chiller.
Gas Chromatograph (Perkin Elmer, Clarus 480) coupled to a FID-ECD detector (Flame Ionization Detector- Electron Capture Detector). The instrument is connected to an autosampler (Autosystem, Perkin Elmer) and is controlled by TotalChrom Software.
Gas chromatograph (Thermo Fisher, Trace 3000) coupled to a mass spectrometry (MS) detector (Thermo Fisher, ISQ7000). The device is connected to an autosampler (Thermo Fisher. AI 1310) and is controlled by a Chromeleon software.
HPLC (quaternary pump, column Oven mod. LC-100 and Micro Pump Series 200, Perkin Elmer, USA) coupled to a fluorescence detector (Perkin Elmer Series 200a). The device is controlled by Chromeleon Software.
HPLC (binary pump, Vanquish TM Core HPLC system, Thermo Scientific TM, Italy) coupled to a high-resolution mass spectrometer (Orbitrap Exploris 120, Thermo Scientific TM, Italy). The device is controlled by XcaliburSoftware (version 5.1).
Spectrophotometer (Biolog Microstation System, Biolog Inc.) for the reading of microplates (96 positions). This instrument is controlled by specific software.
Contact person: Dr. Luisa Patrolecco – luisa.patrolecco AT cnr.it
Brief description
The experimental activities carried out at the MicroChem Lab in Rome focus on the analytical determination of legacy and emerging organic micropollutants and their metabolites, in order to understand their diffusion, distribution, persistence dynamics and fate in the environment. The studies performed at the MicroChem Lab aim also to study the relationships between the climate change and the spread of contaminants at global and local scale. For these purposes, the development and optimization of highly specific and sensitive analytical methodologies for the detection of chemicals at trace and sub-trace concentration levels in the environmental matrices, is required.
Environmental compartments
The experimental activities focus on aquatic and terrestrial ecosystems, therefore the environmental matrices of interest are: Surface waters and sediments (seas, rivers, lakes), snow/ice, soils, aquatic and terrestrial vegetation, biota.
Analytical techniques
The analytical determination of the organic micropollutants is performed by the combination of:
- Pre-treatment methods (e.g. freeze-drying, filtration, etc.);
- Extraction/Clean up methods (solid-phase extraction-SPE, pressurized liquid extraction-PLE, liquid-liquid extraction-LL);
- Sensitive and selective analytical methods based on the coupling of chromatographic techniques (HPLC or GC) with fluorescence, FID-ECD and mass spectrometry detection.
Equipment
The MicroChem Lab is equipped with the following analytical tools:
Benchtop lyophilizer (freeze-dryer LABCONCO) 2.5 L capacity, equipped with a touchscreen display, for the pre-treatment of solid matrices subsequently extracted with PLE.
Solid Phase Extraction (SPE): 12 inlets solid phase extractor connected to cartridges packed with specific adsorbents for the extraction of target compounds from liquid matrices through a vacuum system.
Sonicator (Branson, mod. 2510) for the extraction of chemical compounds from solid matrices by using suitable solvents.
ASE 150 (Dionex, Thermo Scientific) to perform pressurized liquid extraction (PLE) of organic pollutants from solid matrices.
Speed Extractor E-916 (Buchi) to perform simultaneous pressurized liquid extraction (PLE) of 6 samples, operating in different modes.
 Rotavapor R 100 (Buchi), equipped with an electronic interface to control the vacuum system and the recirculating chiller.
Rotavapor R 100 (Buchi), equipped with an electronic interface to control the vacuum system and the recirculating chiller.
Gas chromatograph (Thermo Fisher, Trace 3000) coupled to mass spectrometry (MS) detector (Thermo Fisher, ISQ7000). The device is connected to an autosampler (Thermo Fisher. AI 1310) and is controlled by a Chromeleon software.
HPLC (quaternary pump, column Oven mod. LC-100 and Micro Pump Series 200, Perkin Elmer, USA) coupled to a fluorescence detector (Perkin Elmer Series 200a). The device is controlled by a Chromeleon Software.
HPLC (binary pump, Vanquish TM Core HPLC system, Thermo Scientific TM, Italy) coupled to a high-resolution mass spectrometer (Orbitrap Exploris 120, Thermo Scientific TM, Italy). The device is controlled by XcaliburSoftware (version 5.1).
Contact person: Dr.Luisa Patrolecco – luisa.patrolecco AT cnr.it
 Ministero dell'Universita e Ricerca
Ministero dell'Universita e Ricerca
Programma Ricerche Artico
Programma Nazionale di Ricerca in Antartide
 Ministero degli Affari Esteri e della Cooperazione Internazionale
Ministero degli Affari Esteri e della Cooperazione Internazionale
L'Italia e l’Artico
L’Italia e l’Antartide
CNR-ISP
National Research Council
Institute of Polar Sciences
c/o Scientific Campus - Ca' Foscari University Venice - Via Torino, 155 - 30172 VENEZIA MESTRE (VE)
Phone: +39 041 2348547 - E-mail: protocollo.isp AT pec.cnr.it
Fax: +39 041 2348 549 - Codice Fiscale: 80054330586 - P.I.:02118311006
Unless otherwise indicated, the content of this site is licensed : Attribution Non Commercial Share Alike 4.0 International (CC BY-NC-SA 4.0)
Privacy policy e Cookie policy - Transparent administration (CNR)